
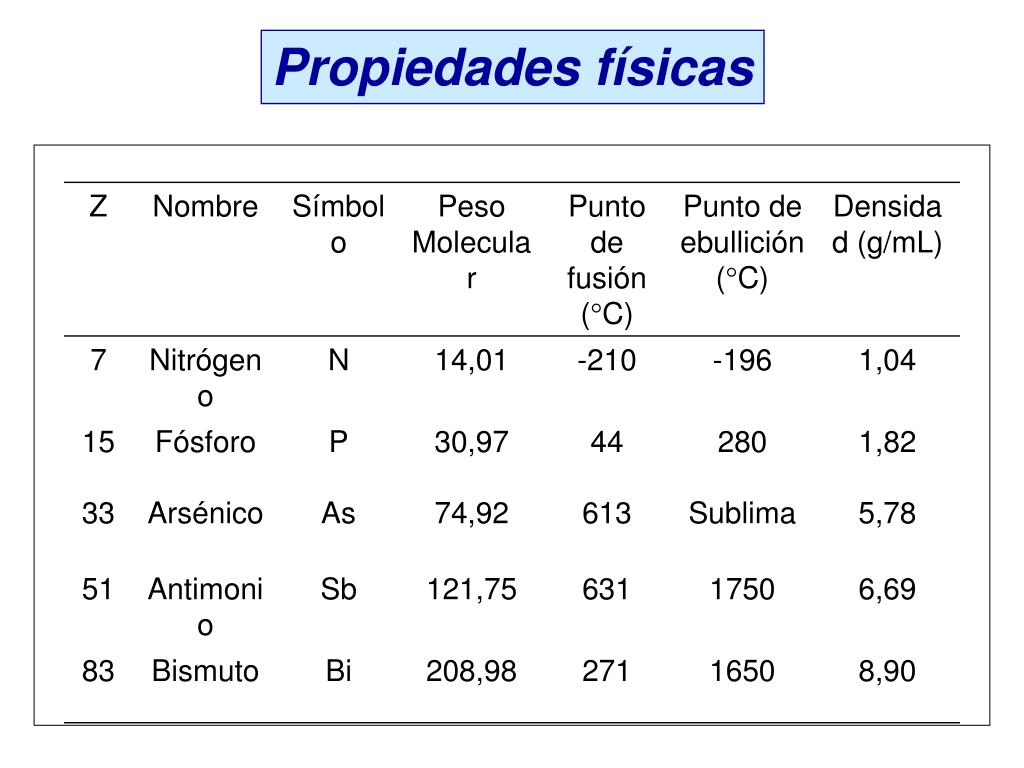
Antimony, atomic number 51, is a silvery. Answer (1 of 3): Nuclear charge is the charge of the nucleus (as one would expect), more importantly effective nuclear charge is the charge exerted by the nucleus on a given electron. Despite the modern IUPAC notation, the Group 15 elements are still referred to as Group V elements in particular by the semiconductor industry. Multiple oxidation states distinguish the p-block elements. Elements in the pnictogen group Nitrogen. We find that an 'extended s' state at the valence band minimum, described alternatively as a cation valence state or a modulated interstitial planewave state, plays a crucial role in. Properties for Group 16 of Periodic Table elements. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Nitrogen is the lightest element in Periodic Table Group 15, also known as the pnictogens. 20 Best selling See all - Best selling Showing slide \)-subshell of their outermost energy level. The pnictogens stability can be depicted using molecular dynamic simulations performed at high temperatures and materials phonon frequencies.
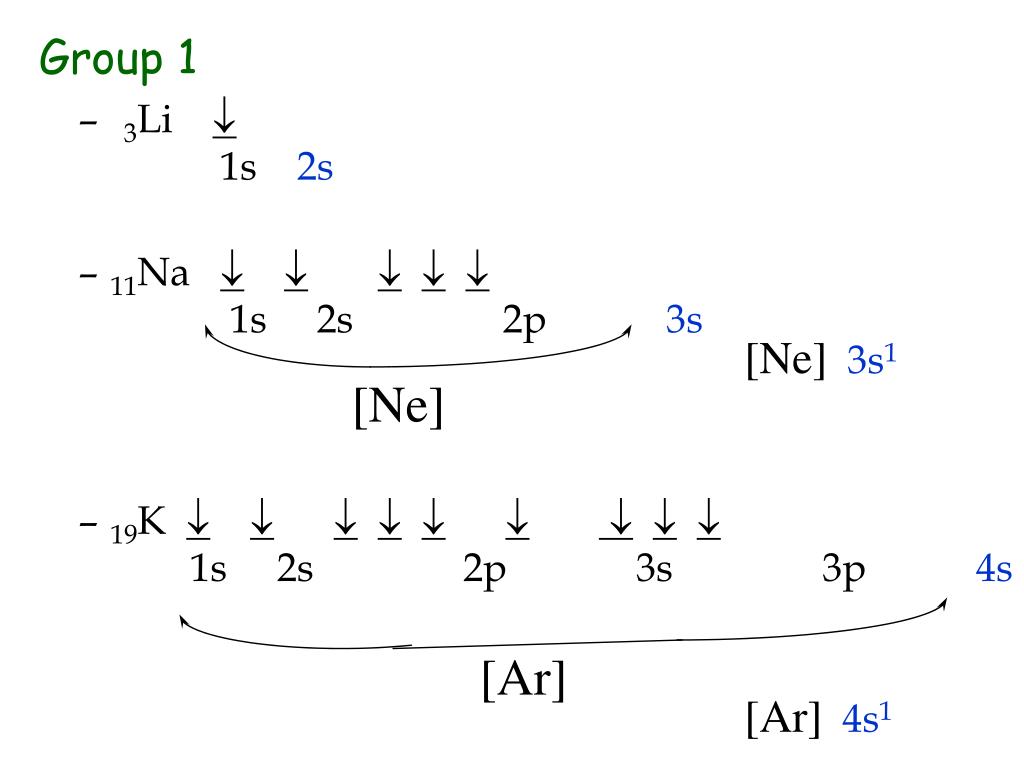
This arrangement is a consequence of the σ‐bonds formed between adjacent atoms due to the half‐filling. The former is a measure for the electronic polariz- Pnictogens have multiple allotropic forms resulting from their ns2 np3 valence electronic configuration, making them the only elemental materials to crystallize in layered van der Waals (vdW) and quasi-vdW structures throughout the group. The oxidation state of group 17 elements (halogens) is -1, while group 18 elements (noble gases) is 0. Topological and thermoelectric properties of double antiperovskite pnictides. Transition Metal Ions The ns electrons are at a higher energy than the (n-1)d electrons so they are always removed before the (n-1)d electrons when TMs form cations.In 1772, a Scottish physician named Daniel Rutherford was the first to discover and isolate it. Because of the precisely half-filled electronic arrangement of the 'n p' subshell, the elements of this group are genuinely steady and stable. There are five electrons in the valence shell of these elements. Nonmetal atoms gain electrons to form anions with a negative charge equal to the A group number minus eight. Thus, the valence shell electronic arrangement for these elements becomes ns2 np3. Year PlayerName Club Total NS1 NS2 NS3 NS4 NS5 NSQ NSS NSF NP1 NP2 NP3 NP4 NP5 NPQ NPS NPF NT1 NT2 NT3 NT4 NT5 NTQ NTS NTF NF1 NF2 NF3 NF4 NFQ NFS NFF 148 2020 NOEL KENNEDY KCC 3 1 1 1. Ion Electron Configurations Metal atoms lose electrons to form cations with a positive charge equal to the group number. I havent played the NP3 yet, so take the above with a grain of salt, but I followed a similar change from Kawais RH3 (two sensors) to RH3-II (three sensors), and the change was quite noticeable. Half-filled d subshells are favored if possible (Hund’s Rule) 3dĮxample 5 What family of elements is characterized by having an ns2np3 valence-electron configuration? Group 5A

#NS2 NP3 PLUS#
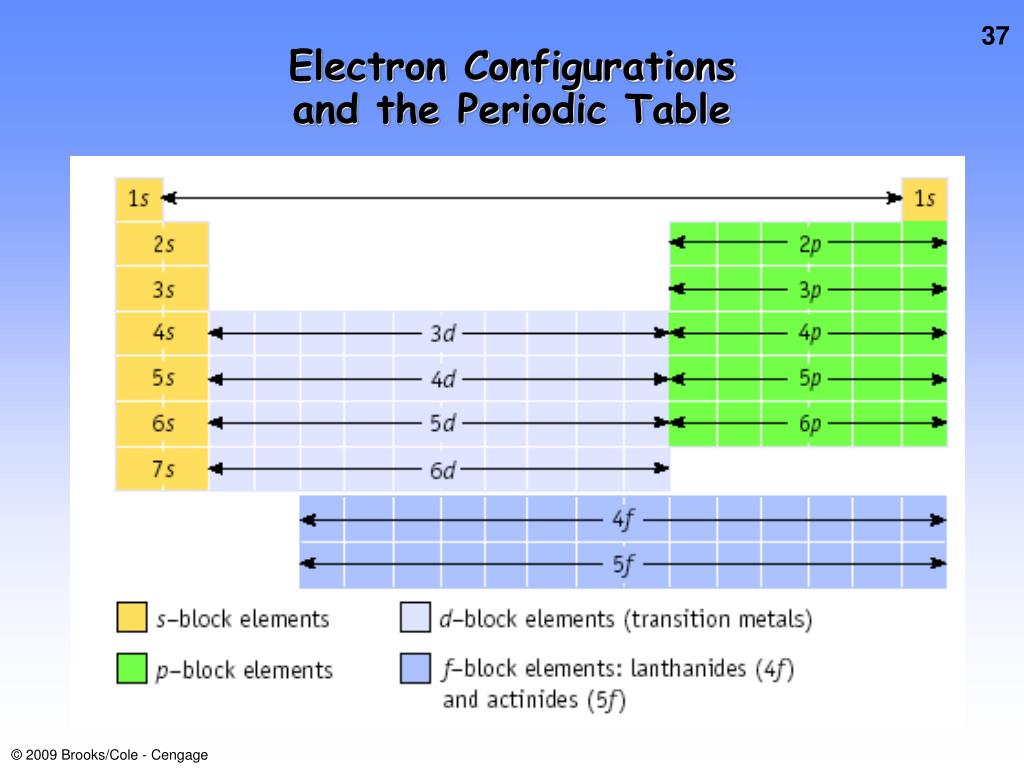
Valence electrons – electrons held in outer shell Core electrons – electrons held in filled inner shellsĮlectron Configurations of Transition Elements ĭ subshell being filled valence electrons - s and p electrons in outermost shell plus electrons in incompletely filled (n - 1)d subshell (n - 1)d orbitals are filled after ns orbitals and before filling np orbitals Carbon, Silicon, Germanium, Tin, and Lead. Hence, elements of IVA or 14 th group have this configuration. Valence Electrons Chemically similar behavior occurs among elements within a group in the periodic table. Solution Verified by Toppr ns 2 np 2 indicates that elements belong to the p-block element having 2 electrons in the outermost shell. go back and pair electrons with 4, 5 and 6 Hund’s Rule – electrons pair only after each orbital in a subshell is occupied by a single electron C: 1s 2 2s 2 2p2 1sĮlectron Configurations and the Periodic Table.half-fill each orbital with first 3 electrons.for atoms in ground state – electrons occupy energy shells, subshells and orbitals that give the lowest energy for the atom Įlectron Configurations for orbitals with same energy (degenerate) such as the three 2p orbitals.Electron Configuration – complete description of orbitals occupied by all the electrons in an atom


 0 kommentar(er)
0 kommentar(er)
